Alcohol answer r oh - Study guides, Class notes & Summaries
Looking for the best study guides, study notes and summaries about Alcohol answer r oh? On this page you'll find 355 study documents about Alcohol answer r oh.
Page 4 out of 355 results
Sort by
-
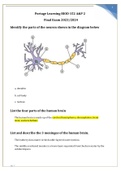
Portage Learning BIOD 152 A&P 2 Final Exam
- Exam (elaborations) • 31 pages • 2023
-
- $10.99
- 2x sold
- + learn more
Portage Learning BIOD 152 A&P 2 Final Exam 2023/2024 Identify the parts of the neuron shown in the diagram below a. dendrite b. cell body c. nucleus List the four parts of the human brain The human brain is made up of the cerebral hemispheres, diencephalon, brain stem, andcerebellum. List and describe the 3 meninges of the human brain. The leathery dura mater is the double-layered outer meninx. The middle arachnoid meninx is a loose layer separated from the dura...

-
Solution Manual for Organic Chemistry Mechanistic Patterns Canadian 1st Edition by Ogilvie Ackroyd Br
- Exam (elaborations) • 811 pages • 2024
-
- $16.99
- + learn more
Chapter 1 Carbon and Its Compounds CHECKPOINT PROBLEMS Practice Problem 1.1 a) S — 1s2 2s2 2p6 3s2 3p4 b) Cl — 1s2 2s2 2p6 3s2 3p5 c) Na+ — 1s2 2s2 2p6 1-2 Copyright © 2018 Nelson Education Limited Practice Problem 1.2 a) Count valence electrons. Build a basic bonding framework and account for electrons used. Add remaining electrons and check for formal charges. The molecule has a lone pair on the nitrogen. All other electrons are bonding electrons. b) Count valence electrons. Build a bas...

-
Solution Manual for Organic Chemistry Mechanistic Patterns Canadian 1st Edition by Ogilvie Ackroyd Br
- Exam (elaborations) • 811 pages • 2024
-
- $19.99
- + learn more
Chapter 1 Carbon and Its Compounds CHECKPOINT PROBLEMS Practice Problem 1.1 a) S — 1s2 2s2 2p6 3s2 3p4 b) Cl — 1s2 2s2 2p6 3s2 3p5 c) Na+ — 1s2 2s2 2p6 1-2 Copyright © 2018 Nelson Education Limited Practice Problem 1.2 a) Count valence electrons. Build a basic bonding framework and account for electrons used. Add remaining electrons and check for formal charges. The molecule has a lone pair on the nitrogen. All other electrons are bonding electrons. b) Count valence electrons. Build a bas...

-
GVSU CHEM 230 (Organic and Biochemistry) Questions With Complete Solutions
- Exam (elaborations) • 5 pages • 2023
- Available in package deal
-
- $10.49
- + learn more
NH2 functional group correct answer: amino RNH2 correct answer: primary amine R2NH correct answer: secondary amine R3N correct answer: tertiary amine bronsted-lowry acid correct answer: proton donor bronsted-lowry base correct answer: proton accepter lewis base correct answer: electron donor lewis acid correct answer: electron accepter alkyl halide correct answer: a compound that has an alkyl group bonded to a halogen atom, R-X thiol correct answer: has -SH g...

-
MCAT AAMC practice exam Chem Phys with Complete Solutions
- Exam (elaborations) • 34 pages • 2024
-
- $13.49
- + learn more
MCAT AAMC practice exam Chem Phys with Complete Solutions What quantity of Compound 1 (483.5g) must be provided to prepare 100.00 mL of solution with a concentration equal to Ki (60.3uM)? -Answer-M=mol/v 1 umol=0.000001 moles (1x10^-6) 2.92mg If only [I] is increased, then [ESI] or [EI] increases. This is an example of: A. the Bose-Einstein Principle. B. the Heisenberg Uncertainty Principle. C. the Le Châtelier's Principle. D. the Pauli Exclusion Principle. -Answer-Solution: The corr...

-
All Domains Jean Inman Questions with Explanations of Answers 2022/2023
- Exam (elaborations) • 269 pages • 2022
-
- $8.99
- 9x sold
- + learn more
All Domains Jean Inman Questions with Explanations of Answers Which amino acids must be present in a parenteral solution? a. valine, leucine, glycine, alanine b. tryptophan, phenylalanine, threonine, isoleucine c. phenylalanine, methionine, arginine, tyrosine d. lysine, glutamic acid, alanine, glycine - ANS- b. tryptophan, phenylalanine, threonine, isoleucine A parenteral solution requires adequate supplementation of all essential amino acids. Essential amino acids = those that can...

-
PORTAGE BIOCHEMISTRYMODULE 1 EXAM | QUESTIONS & ANSWERS (VERIFIED) | LATEST UPDATE | GRADED A+
- Exam (elaborations) • 16 pages • 2024
-
- $11.99
- + learn more
1 PORTAGE BIOCHEMISTRYMODULE 1 EXAM | QUESTIONS & ANSWERS (VERIFIED) | LATEST UPDATE | GRADED A+ Definition of biochemistry Correct Answer: chemistry of life; how properties of organisms relate to their molecules What are the 3 things biochemists study? Correct Answer: 1. The relationship between structure and function of biomolecules 2. Chemical reactions of organisms (metabolism) 3. Communication within and among organisms What are bulk elements? Correct Answer: Elements that a...

-
CHEM 210 Final Exam Study Guide Graded A+
- Exam (elaborations) • 24 pages • 2023
-
- $13.49
- + learn more
CHEM 210 Final Exam Study Guide ***exam 1 final review*** What are the differences between aliphatic (including alkanes, alkenes,alkynes and cyclic compounds) aromatic hydrocarbons are. - correct answer ALIPHATIC Hydrocarbon: Non- aromatic -Alkanes & CycloAlkanes:single bonds; saturated:Contain only carbon and hydrogen & C-C & C-H single bonds -Alkenes:double bond(s) -Alkynes: triple bond(s) *alkenes and alkynes are unsaturated: at least one C-C double or triple bond(s) AROMATIC H...

-
MODULE 8 PORTAGE 219 ORGANIC CHEMISTRY EXAM | QUESTIONS & ANSWERS (VERIFIED) | LATEST UPDATE | GRADED A+
- Exam (elaborations) • 21 pages • 2024
-
- $14.49
- + learn more
1 MODULE 8 PORTAGE 219 ORGANIC CHEMISTRY EXAM | QUESTIONS & ANSWERS (VERIFIED) | LATEST UPDATE | GRADED A+ large molecule made by repetitive linking of smaller units (monomers) Correct Answer: Polymer very large molecule composed of thousands of covalently bonded atoms (ex: polymer) Correct Answer: Macromolecule two ways polymers are made Correct Answer: 1. natural (in nature) 2. synthetic (in lab) natural polymers Correct Answer: rubber, carbs: starch & cellulose, proteins, nu...

-
MCAT AAMC practice exam Chem Phys Exam Questions and Answers 2024
- Exam (elaborations) • 23 pages • 2024
- Available in package deal
-
- $13.49
- + learn more
MCAT AAMC practice exam Chem Phys Exam Questions and Answers 2024 What quantity of Compound 1 (483.5g) must be provided to prepare 100.00 mL of solution with a concentration equal to Ki (60.3uM)? -Answer-M=mol/v 1 umol=0.000001 moles (1x10^-6) 2.92mg If only [I] is increased, then [ESI] or [EI] increases. This is an example of: A. the Bose-Einstein Principle. B. the Heisenberg Uncertainty Principle. C. the Le Châtelier's Principle. D. the Pauli Exclusion Principle. -Answer-Solution: ...

Study stress? For sellers on Stuvia, these are actually golden times. KA-CHING! Earn from your study resources too and start uploading now. Discover all about earning on Stuvia


